The SEEQ™ MCT (mobile cardiac telemetry) System is an external heart monitor that can be prescribed for up to 30 days to help detect and diagnose the cause of irregular heartbeats in patients. This FDA approved, CE marked, water-resistant patch provides near real-time continuous arrhythmia detection for up to 7.5 days without having to replace or recharge the battery. This device was sold in the U.S. market from 2010 to 2018. This device can be expanded for use in remote patient monitoring – tracking and reporting in near real-time: heart rate, respiratory rate, fluid levels, body temperature, and patient activity.
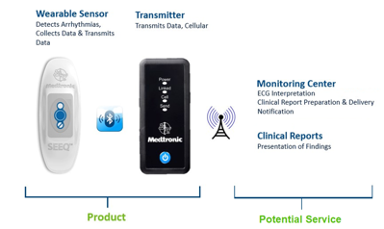
On behalf of our client, Medtronic, yet2 announces that a SEEQ Mobile Cardiac Telemetry Patch and Bluetooth/cellular automatic data collection and transmission technology, and related business assets are available for acquisition.
The SEEQ product (originally named Nuvant) was conceived, designed, developed and launched by Corventis, Inc. of San Jose, CA. The underlying sensor technology, Piix, received FDA approval in 2009 for detect signs of heart failure by monitoring heart rate, respiratory rate, temperature, fluid levels and patient activity. The Nuvant Mobile Cardiac Telemetry System which uses the Piix sensor, was cleared by the FDA in 2010. Medtronic acquired Corventis in May 2014, rebranding the Nuvant MCT as SEEQ MCT. The SEEQ MCT business achieved $10M in sales during FY 2017 before being discontinued in May 2018.
Medtronic believes the properties, features and benefits of this technology may be of great interest to others wishing to offer an FDA approved, turnkey mobile cardiac monitoring and solution to their client base.
Market Needs Addressed
SEEQ Mobile Cardiac Telemetry (MCT) provides continuous, near-real-time ECG (electrocardiogram) monitoring of a patient for up to 30 days. Other forms of monitoring require a patient to wear a device which must be returned to the doctor before the recorded information can be reviewed. This creates a significant time lag time, up to 20 days, between an event occurring and a doctor seeing it. MCT is useful for uncovering causes of palpitations or undiscovered arrhythmias, transient chest pain (angina), shortness of breath (dyspnea), dizziness (syncope), sleep disorders, and other non-life-threatening cardiac disorders that could benefit from 24-hour monitoring. Several studies (DOI: 10.1109/EMBC.2012.6346457; DOI: 10.2147/MDER.S54038) have shown that MCT to be significantly more likely to observe and diagnose arrhythmia than Holter or event monitors. Reasons cited include (1) the first incidence of clinically relevant arrhythmias often present themselves in excess of 2 days, the typical prescription time of a Holter monitor; (2) better patient compliance due to comfort and usability.
SEEQ MCT transmits cardiac information to a cloud portal which can be accessed by a monitoring center (Independent Diagnostic Testing Facility or in-house),via zLink, a dedicated smart cellular device which relays data securely and privately from the patch without relying on a patient’s smart device or cellular plan. At the monitoring center, either an in-house or an Independent Diagnostic Testing Facility, the data is reviewed and annotated by medical technicians, summarized into reports and subsequently made available to the physician.
IP Portfolio
Medtronic has filed worldwide for patent protection for multiple patent families (25 assets in total). The US patents from the key families are listed below.
| Patent | Type | Short Description |
| 9,084,583 | Device | Battery + Circuitry + at least 2 electrodes + 2 switches |
| 8,897,868 | Device | Battery + Circuitry + at least 2 electrodes + 2 switches + separate lead that measures impedance b/w 2 electrodes (DC – on adhesion and effect on impedance) |
| 9,411,936 | System (Bluetooth) | Device + Gateway (device on belt) + Server(patient security, privacy, match with patient registered on |
| 9,445,719 | System | Whole System on Monitoring plurality of patient devices |
| 8,823,490 | System | Adds in adherents, alerts, battery, etc. |
| 9,848,779 | Device | 2 Parts: Disposable + Reusable |
| 9,636,069 | Device | Describing what kind of AF trying to monitor |
| 9,706,938 | System | Describing monitoring PVCs |
Technology Description
The SEEQ™ MCT (mobile cardiac telemetry) System is an external heart monitor that can be prescribed for up to 30 days to help detect and diagnose the cause of irregular heartbeats in patients.
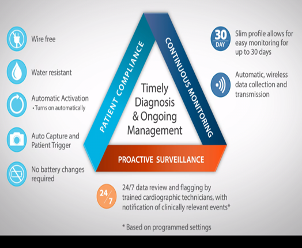
The Medtronic technology offers numerous potential advantages, including the following, among others:
- Comfortable and lightweight device (50g, 160x60x15mm) improves patient compliance.
- Long-life battery in patch operates for 7.5 days
- Proprietary adhesive system provides better water-resistance, longer wear, and more reliable data collection over other products in the market.
- Sophisticated patented internal DSP algorithms detect tachycardia, bradycardia, pause, atrial fibrillation and other arrhythmias with a single lead device.
- Captures and continuously transmits cardiac data. Integrated trigger button allows patients to notify their doctor when they experience any symptoms.
- Near real-time data from the wearable monitor is sent via Bluetooth to a cellular transmission device which forwards the cardiac data to a portal or Independent Diagnostic Testing Facility (IDTF) in near real time. Reports on the data can be made available online to physicians 24/7.
- Key diagnostic information reviewed by doctor in days, not weeks.
- Automatic activation – can be applied easily by patient at home, no skin abrasion or special skin preparation is required.
- Single ECG channel with sampling rate of 200 Hz, digital resolution of 16 bits, and heart rate measurement range between 25 – 250 bpm.
- QRS Detection (average) – Sensitivity AHA: 98.95%, MIT-BIH: 99.83%; Positive Prediction Value: AHA 99.34%; MIT-BIH: 99.84%
- AF Duration (gross) – Sensitivity MIT-BIH: 90%; Positive Prediction Value: MIT-BIH: 85%
- Transmitter automatically relays sensor data to monitoring center. Receives wearable sensor data up to 30 feet away. Battery life is up to 12 hours.
Technology and Product Development and Other Assets
SEEQ/Nuvant Mobile Cardiac Telemetry was a fully operational business from 2010 to 2018. Assets include aforementioned patents, trademarks, manufacturing know-how, and trade secrets.
The business includes technology, know-how and patents around: (a) Mobile Cardiac Telemetry device (b) collection of heart rhythm data, (c) DSP derived probabilistic algorithms to detect arrhythmias, (d) Bluetooth wireless transmission of the data from SEEQ MCT to zLink, a cellular transmission device which securely and privately sends data to a (e) web portal where episodes, patient journey, notifications, status progress, reports, etc. can be monitored by the physician via a dashboard. The portal also records necessary information for billing purposes.
The technical annotations, customer service functions to confirm insurance coverage, billing and reimbursement elements of the business model are not available or included in the business assets to be sold.
Partnership Opportunities
Medtronic is open to a variety of types of licensing or asset sale deal structures. Initial preference is to sell or license the technology to a single company capable of producing, integrating, and broadly commercializing the SEEQ MCT product.
We believe that prospective acquirers will have strategic reasons to want to bring a differentiated arrhythmia monitoring device to market relatively quickly.
- Medical device companies that have health monitoring products that are considering entering the ambulatory arrhythmia monitoring business, or seeking to extend/improve their arrhythmia monitoring product line up.
- Independent Diagnostic Testing Facilities or AI/ML based Diagnostic Software Companies that are seeking to offer product as well as services
- Telemedicine and Home Healthcare Service Providers that are seeking innovative ways to monitor patients.
- Physiological Monitoring Solution Providers seeking to augment their offerings with a cardiac monitoring device.
Licensees can expect to receive excellent support from technologists familiar with the materials, algorithms, and required processes, learning nuances of process and formulation variables and performance. Medtronic has excellent experience, know-how and credibility in the design, development and manufacture of cardiac monitoring devices.
Company Overview

Medtronic is among the world’s largest medical technology, services and solutions companies – alleviating pain, restoring health and extending life for millions of people around the world. Medtronic employs more than 90,000 people worldwide, serving physicians, hospitals and patients in more than 150 countries. The company generated $30 billion in revenue in FY2018.
Further Information
Additional information not subject to confidentiality requirements will be provided to prospective licensees/acquirers having credible interest.
Extensive additional information may be made available to qualified interested parties under the provisions of a confidential disclosure agreement.
Tags: Medical Device, MedTech
