Overview:
This client is seeking a contract development and manufacturing company (CDMO) that can produce a professional oral care product, containing an active formulation, packaging, and delivery component.
Background:
This client is looking to develop a generic version of an existing product to treat patients with adult periodontitis (gum disease). The product they are looking to produce is a generic of a professional oral care product currently on the market and used in combination with scaling and root planing (SRP) procedures by dentists.
Constraints:
- CDMO must be able to manufacture at least one of the three components:
- The formulation: Minocycline HCl delivered in Microspheres, 1mg
- Microsphere is bioresorbable polymer, Poly (glycolide-co-dl-lactide) or PGLA, providing sustained release
- Microspheres 30-120 µm in diameter
- Capabilities on microsphere formulation and sustained release of the antibiotic over time is highly desired
- The Unit Dose Cartridge
- The hand-held Thumb-Ring delivery device
- The formulation: Minocycline HCl delivered in Microspheres, 1mg
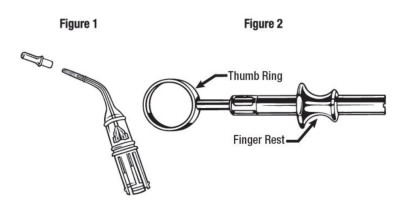
A diagram showing 1) the cartridge and 2) a spring-loaded cartridge handle mechanism. In the Arestin system, the delivery device is made of metal, reusable, and able to be sterilized. The proposed generic drug product is not required to have the same container closure system (CCS) as the RLD. However, the ANDA generally must contain information to show that the proposed generic drug product has the same conditions of use and the same labeling, with certain permissible differences, as the RLD. Refer to FDA guidance for industry Determining Whether to Submit an ANDA or a 505(b)(2) Application (May 2019).
- CDMO must have the capability to conduct the stability aging
- CDMO should provide comprehensive data to demonstrate the proof of “equivalence” to the RLD (Reference Listed Drug)
- CDMO must have the manufacturing capacity to produce at least 50k units annually and up to 1M+ units annually within required time frame.
- CDMO must have safety and quality certifications such as GMP.
- CDMO has an active site registration with regulatory agencies (must be registered with the US FDA).
- For Brazil, the manufacturing site must have a Good Manufacturing Practices certificate, issued by the Brazilian regulatory agency. Additionally, If the site is located in Brazil, it must have sanitary license and operating permission issued by the local regulatory agencies.
- For Europe, the product needs to be produced by a EU GMP compliance site and a drug development plan covering clinical efficacy and safety is required. If located outside the EU or the UK, batch release and testing is necessary in the EU and the UK, respectively.
Possible Solution Areas:
- Pharmaceutical CDMOs
- Manufacturing And Packaging CDMO
Related Links
- Seeking: Untraditional Oral Care Product Formats
- Seeking: Novel Delivery Systems & Packaging Solutions for Oral Care
- Scouting’s hottest trend: Contract Manufacturing Organizations
- Seeking: Minority-owned Suppliers in Beauty and Personal Care
Photo by Benyamin Bohlouli on Unsplash